FAQ
This section contains answers to Frequently Asked Questions regarding the survey.
If this information does not fully answer your questions, please feel free to contact any partner.
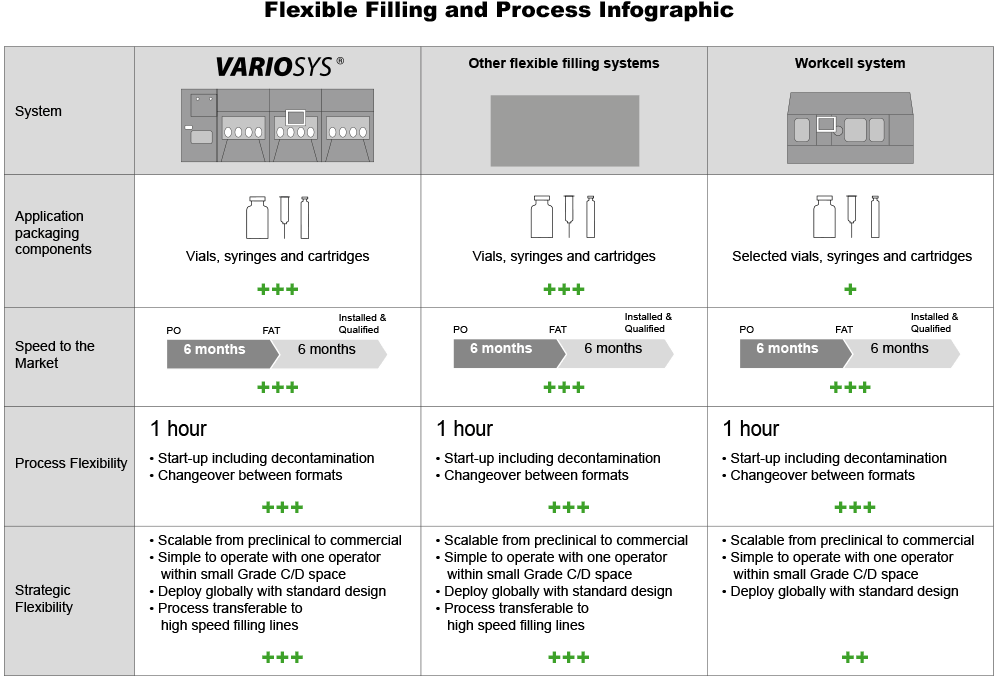
VarioSys® is an all-in-one system designed to provide maximum flexibility in pharmaceutical production. It is suitable for a wide range of container types and applications, giving you the flexibility to produce clinical samples and small commercial batches with a single aseptic filling line. Machine modules by Bausch+Ströbel can be securely interlinked with a standard SKAN isolator system. The “lock and key” principle of VarioSys® allows modules to be interchanged any time depending on production requirements.
The range of pharmaceutical production applications can be extended by adding a state-of-the-art GEA freeze dryer making VarioSys® into a complete system which meets all FDA requirements for pharmaceutical production. Every partner in our conglomerate has extensive experience and can offer advanced technologies tailored to suit your exact requirements.
Why is the L-flange called L-flange?
The Pharmaceutical Safety Isolator with L-flange (PSI-L) is a modular isolator system with a unique
L-shaped flange designed specifically for VarioSys®. It allows process equipment to be exchanged very quickly. The short decontamination cycle and the optional return air filter (FIPA) make it a universal sterile workspace for aseptic, toxic and/or potent pharmaceutical products.
Which types of container can be processed?
VarioSys® can be used for processing vials, syringes and cartridges made of glass or plastic. The system is approved for Ready to Use (RTU) containers by BD, Schott, Ompi and West. It may also be possible to process other types of RTU containers. Please contact us for further information.
Is VarioSys® also available for nested and bulk processes?
Yes. Bausch+Ströbel production modules are designed for filling and closing all primary packaging components commonly used in the pharmaceutical industry. VarioSys® has been specifically designed for optimal handling and processing of RTU containers. The system can easily be configured to process bulk components using a standard B+S rinsing machine and depyrogenation tunnel. Combined lines (nested and bulk) are also possible.
Can semi-automatic and table-top machines also be used on a VarioSys® module?
Yes. Semi-automatic and table-top machines (resistant to VHP) are available for the following applications:
- Filling containers with exact amounts of liquid and powder product
- Closing containers
How does the discharge of bulk components work?
The discharge system is a mobile single-tray loader. The tub discharge track and the bulk discharge track can be easily interchanged for maximum flexibility.
How does the discharge of tubs work?
The tubs exit the isolator via a standardized “mousehole” along a mobile roller track with reject discharge capability. The tub discharge track and the single container bulk discharge track can be easily interchanged for maximum flexibility.
What is the overall height/footprint of the machine?
Width of isolator (per PSI-L): 1.90 m
Depth of isolator with B+S module: 1.50 m
Height of isolator: 3.00 m (less than 10 feet)
No additional space above the clean room for auxiliary equipment is usually required. The machine modules and the PSI-L are self-contained.
Can the VarioSys® be moved to different locations, lines and countries?
Yes. Modules can be transported to different locations, lines and countries worldwide.
Thanks to the standardized isolator size and electrical infrastructure (VSE90100), modules can be exchanged anywhere in the world.
In which class of cleanroom can VarioSys® be installed?
Cleanroom classes ISO 7 and 8 (classes C and D) are possible. The lyo chamber can also be installed in a class ISO 7 cleanroom. The lyo chamber has an ISO 7 surround housing for all-round line access.
Is VarioSys® also available from other isolator or freeze dryer vendors?
No. VarioSys® is only available from our selected and trusted partners.
How many operators are required to operate a VarioSys® line?
Depending on batch size and process type, the number of operators varies from a minimum of 2 to a maximum of 4.
The number of operators can be reduced from 4 to 2 by using our automatic de-nester (DDM9105) or a rinsing machine/tunnel as a feeding machine in combination with our bin and feed unit (BZV8110).
Is the rear wall freely configurable? Is the machine accessible from both sides?
The rear wall is designed for process-specific installation of RTP ports (e.g. product supply, product circulation, stopper and cap supply).
How many gloves are needed per PSI-L module?
According to mock-up studies, four glove ports are recommended at standardized and ergonomic working positions.
Is VarioSys® available without glove ports?
To minimize product loss, glove ports are essential for trouble-shooting. A provision for non-permanent gloves is optional.
How long does it take to exchange a module?
It takes approx. 30 minutes to exchange a module, e.g. to switch from vial processing to syringe processing, not including the time required for a VHP cycle.
How long does it take to reconfigure VarioSys® for filling the same vial using the same closures but a different product?
It takes approx. 10 minutes to change over the product paths.
Can the machine handle a nitrogen atmosphere for products that are oxygen-sensitive?
There are two options: a nitrogen blanket in combination with dispensing needles or a nitrogen filled chamber.
Can the modules be operated outside the isolator?
Production outside isolator is not possible for safety reasons. Power-up and jog button operation are possible outside the isolator. A special provision or docking station is needed.
Cross-contamination
There is a risk of cross-contamination even after VHP cycles. With VarioSys®, however, it is possible to prevent cross-contamination by using a separate module for each product (dedicated customer/patient/product modules). CMOs use separate, dedicated modules for each customer.
What is the VSE90100?
The VSE90100 is a supply and control unit which supports up to 4 VarioSys® modules:
It is the central infrastructure cabinet for all modules.
- Central HMI
- Central power supply
- Central vacuum supply
- Central safety monitoring
Where is the VSE90100 located?
The VSE cabinet can be located inside a cleanroom or in an outside/mezzanine area (technical area). Cable lengths of up to 35m (100 feet) are possible.
How are the modular machines supplied with power?
Coded power plugs can easily be connected on the rear side of the module or L-flange. Power comes from a centralized supply and control unit or VSE. All connections are standardized for easy plug and play use.
What are the required utility connections (in addition to electrical power)?
Rinsing machine: WFI, compressed air
Tunnel: compressed air and chilled water
L-flange modules (depending on type): vacuum (a pump is supplied), compressed air and nitrogen
Can the filling and closing systems used on VarioSys® be upscaled at a later date?
The filling, stoppering, crimping and control systems used on the VarioSys® are fully scalable for use in high-speed production lines. All batch parameters are transferable and applicable to high-speed production. Tried-and-tested equipment is used to guarantee careful and reliable filling and closing.
How do you access the modules for cleaning and maintenance?
VarioSys® offers 360° access and allows modules to be removed from the isolator for easy cleaning and maintenance outside the isolator.
What is the preventative maintenance schedule for the machine? Could you specify who needs to do it and how often?
Cleaning and maintenance intervals depend on customer requirements and/or specifications. Various maintenance plans are available. Please consider that maintenance can also be done offline (outside of the isolator) while production continues on another module.
100% IPC is the state of the art in advanced filling systems with multiple applications. It allows vials to be processed without any loss of speed during production. This feature can include automated weight control and adjustment as well as smart algorithms for intelligent dynamic filling systems. The batch report can also include data for each custom filled container. This all but eliminates product loss at start-up and when running empty.
It is a huge advantage to use 100% IPC as it saves product and ensures that the process is 100% reliable. The filling system (especially rotary piston pumps) is very precise and capable of running at a reduced IPC rate. 100%IPC at full speed is possible with vials, cartridges and syringes.
What is Advanced Fill?
Advanced Fill avoids product loss and rejects during the start- phase and eliminates the need for emptying the vial filling module. A filling system with Advanced Fill produces no rejects until the very last vial.
Is VarioSys® available for powder filling?
Yes. VarioSys® is available for powder filling into vials using an auger dosing system. All filling modes and Advanced Fill also comes as standard with IPC for powder filling.
Which dosing principle is used for powder dosing?
B+S uses auger dosing, which is very precise. Dosing volume >25mg.
Is powder and liquid filling possible on the same module?
Yes. Powder and liquid filling are possible on the same module.
Which filling systems are available?
- Powders: Auger dosing system
- Liquids: Rotary piston pump filling system, peristaltic pumps, or a time/pressure filling system
- Powder and liquid products can be processed on the same module
- Multiple filling systems can be used on the same module
Is there the capability for a Clean in Place/Sterilize in Place (CIP/SIP) system?
Yes - for liquid and/or powder filling.
How is the machine decontaminated after a run?
A wipe down and VHP cycle is used for non-toxic applications. In the case of toxic/potent applications, a cleaning study is typically part of the project. This can include a wash down and safe filter change procedure.
Is Wash in Place (WIP) possible?
Yes. Our machines are designed for manual WIP with spray guns.
What type of peristaltic pump is used?
The proprietary B+S single-hose peristaltic pump is used. Please contact us for further information.
Is gas flushing possible?
- KSF 5105 filling and closing machine: gas flushing after filling and during closing
- SFM 5105 filling and closing machine: gas flushing during filling and during closing
- SFM 5205 filling and closing machine: gas flushing during filling and during closing
- A full nitrogen environment is also possible.
Yes. Anti-vibration compensation (AVC) technology is used to reduce vibration that might affect the performance of weigh scales.
Is it possible to have the filling system outside the isolator?
Yes. A peristaltic filling system could be installed outside of the isolator for improved access.
How does a disposable fluid path work?
Located on the outside of the isolator, the disposable product path is connected to the product tank at site via a sterile connector. During the course of the project B+S will work hand-in-hand with the customer’s supplier to determine the best possible configuration.
Is filling of toxic/potent product possible?
Yes. Different pressure zones are used for toxic product applications. A negative pressure within the isolator ensures that there is no “leakage” of toxic product. The SKAN PSI-L is equipped with a special H13 safe-change filter system for air return and a drainable gasket for the L-flange.
The product supply system can be customized to meet product requirements. 60 shore silicone hoses by Saint Gobain are recommended.
Can product be recirculated within the product path?
Yes. The rear side of the L-flange can, for example, be equipped with two Sartorius SART ports for product recirculation.
What is a BZV8110 / Is automatic feeding of caps and stoppers possible?
BZV8110 is a portable vibrating system that accepts RTU or Ready-to-Sterilize (RTS) bags and dispenses components into the bags for processing. It is a small unit that improves ease of work and ergonomics while reducing manpower requirements.
How does the feeding of sealing components work?
BZV8110 supports feeding of product from pre-sterilized bags through RTP ports. Sartorius Biosafe ports are preferred because they permit handling from outside the isolator.
Is it possible to have capping on the same module as filling and stoppering?
Yes. In a CFD study we verified that no air from the capping process reaches the open vials. The data from the CFD study are available on request.
Is capping possible inside an isolator?
Yes. Capping is possible in combination with filling and stoppering (KSF5105), either on the same module or on a separate module (KS4105). Capping is also possible in combination with filling on the same module. Caps must be delivered in sterilized Beta bags.
Is it possible to verify a stopper gap on the capping machine?
Yes. A standard stopper height verification system is available. Furthermore we can offer a vision sensor for stopper gap detection before crimping.
Is re-stoppering of vials, syringes and cartridges possible with VarioSys®?
Yes. Our Zero Reject Principle™ makes re-stoppering possible and is standard on the vial filling and stoppering module. The speed of the machine will be reduced by re-stoppering.
Is cap inspection possible?
Yes. The presence of crimp caps can be checked on the KSF5105 filling and closing machine.
What type of RTP ports are used on the VarioSys®?
Sartorius Biosafe outside opening Ø 110mm (preferred)
Getinge DPTE® Alpha Ø 105mm and Ø 190mm
Other suppliers are possible, too.
The typical height, width and depth of a VarioSys® isolator module is 2950mm tall x 1950mm wide x 1320mm deep (116” H x 77” W x 45” D). In addition, there is a control panel which can be mounted on equipment or standalone (same height, 520mm W or 20.5” W) and a modular SARA (Safe and Rapid Airlock) which is 905mm wide (35.6” W). Each of these modules can also be disassembled for initial entry into a room or for access via service elevators.
Are HVAC skids used in addition to the isolator shell?
Typically, all of the requisite HVAC components, including the hydrogen peroxide decon system, are built in and fully integrated with the isolator shell and footprint. For some special applications involving extreme temperature, RH or inert gas requirements, supplemental HVAC components or skids may be added to the system and may be located in an adjacent space or directly above the target isolators.
Do I need a separate H2O2 generator to decontaminate my isolator?
No. The SKAN hydrogen peroxide bio-decontamination system SKANFOG® is fully integrated with the isolator system. No separate control system or equipment is required, and SKAN provides a full package of safety, testing and qualification options for the isolator and bio-decontamination system.
Does the isolator have unidirectional airflow?
Yes. The SKAN isolator utilizes unidirectional airflow to create a fully compliant Grade A / ISO 5 environment. The basic design provides for a 0.45m/s (90 ft/min) airflow to sweep over the process and eliminate or minimize particulate generation. Extensive research and CFD (computational fluid dynamics with computer modeling) studies have been performed for the isolator and process equipment designs, as well as live, in-situ video recorded air flow studies to demonstrate excellent unidirectional flow.
Is the patented NANOX® catalyst available for use with the VarioSys® isolator?
SKAN’s unique and patented catalyst is already built in with VarioSys® and offers a number of major advantages. Primarily, this catalyst allows the isolator to be installed in the room and exhausted without any outside exhaust vent to the roof. This saves tens of thousands of dollars in expensive installation costs and facility modifications. Also, during the aeration portion of the decon process, when a typical isolator consumes hundreds to thousands of cubic feet or cubic meters per minute of room air vented to the outside, the SKAN isolator safely returns that pre-conditioned air back to the room, thereby maintaining the appropriate pressure balance within the cleanroom (no wide-range dynamic balancing required). Finally, this reduces not only the facility installation and control costs; it also minimizes utility costs and is a “green” and environmentally safe solution.
How long is the bio-decontamination cycle for the isolator? What about the SARA (Safe and Rapid) airlock?
The main isolator system decon cycle for a typical cycle with regular aeration to <1ppm concertation is approx. 1 hour and longer for lower levels of concentration. With the new SKANFOG® decon process coupled with the NANOX® catalyst, <60 minutes for a full 10E6 BI kill level and aeration down to <1 ppm is expected, depending upon the process equipment and load conditions.
The typical cycle in the SARA airlock (which is an optional system that can be used to introduce materials or components into main isolator) is approx. 15 minutes for a full 10E6 BI kill level and aeration down to <1 ppm.
Can the VarioSys® isolator be used for other aseptic processes or must you use it only with a filler or lyo?
Yes. The SKAN VarioSys® isolators are very flexible and can be used for most aseptic processing steps from cell therapy and compounding to specialized filtration, bioprocessing or aseptic drug-device assembly. Many manual or automated processes can be integrated with the L-flange with our partners’ process equipment and customized for most aseptic processes.
Can a process tank and/or filtration skid be integrated with the L-flange system?
Yes, product tanks and process piping can be built-into the L-flange system with permanent / welded connections as well as other novel integration methods with seals (gaskets and inflatable) that allow you to remove the tank or other vessel for cleaning and autoclaving out of place and/or CIP/SIP (Clean in Place/Steam in Place) along with the process piping.
Can I use a VarioSys® isolator for potent compounds, ADCs, cell therapy or other special manufacturing substances or situations?
Yes. These isolators are VERY flexible and can be adapted for many manufacturing applications. L-flanges have been adapted with incubators and other chambers for cell culture applications and the isolators can be used under positive or negative pressure with additional return air filter systems for “safe-change” HEPA filtration for containment of potent compounds, even of Band 4-5 materials (low microgram or nanogram level containment). Consult your SKAN containment expert for sample applications and a process risk assessment.
Is Wash in Place (WIP) possible?
Yes. Our machines and the isolator are designed for manual WIP using spray wands. Depending on the configuration different levels of integration can be selected such as special wash- and drying modes.
Is gas flushing possible?
- KSF 5105 filling and closing machine: gas flushing after filling and during closing
- SFM 5105 filling and closing machine: gas flushing during filling and during closing
- SFM 5205 filling and closing machine: gas flushing during filling and during closing
- A full nitrogen (or other neutral gas) environment is also possible with a low oxygen processing environment for preservation of fragile or solvent-based materials in filling or compounding applications. Existing applications have gone as low as 2-5% oxygen (typical ambient concentration is 21% O2) but lower may be possible depending upon the application and gas consumption requirements.
Is environmental monitoring included with the VarioSys® isolator?
Depending upon the application and process, SKAN or our partner process equipment suppliers provide the integrated particle counters, active viable air sampling systems and/or passive viable air sampling equipment. In any case, SKAN can provide a unique valving system to draw hydrogen peroxide decontamination agent through the lines of the isokinetic probes and air sampling units to ensure that there are no dead-legs unexposed to the H2O2 decon process.
Who will validate my VarioSys® isolator?
SKAN works hand-in-hand with our equipment partners to provide a comprehensive validation package utilizing best practices from each process equipment supplier. For the isolator, SKAN provides all GAMP (Good Automation Manufacturing Practices) documentation in addition to a thorough IQ/OQ (Installation and Operational Qualification) and CD/MBQ (Cycle Development / Microbiological Qualification) process with options for basic and extensive qualification packages. SKAN’s cycle development and microbiological qualification methodology is cited as a reference in the 2004 FDA Aseptic Guidance document.
Is it possible to use scales on a mobile module and within the isolator with unidirectional airflow?
Yes. Anti-vibration compensation (AVC) technology is used to reduce vibration that might affect the performance of weigh scales.
The lyophilizer chamber/condenser is uniquely designed to have a small footprint and is completely surrounded by a smooth and cleanable stainless steel enclosure. This means that the lyophilizer chamber inside this enclosure can be placed inside a Class C cleanroom. The lyophilizer ALUS™ (Automatic Load/Unload System) is mounted within the PSI-L isolator using the L-flange coupling system. This class C cleanroom design allows modular equipment on either side of the lyophilizer to easily be exchanged into and out of the PSI-L isolators with no interference from the lyophilizer. Since the lyophilizer is located within the cleanroom, there is full 360° access right around the production line in the class C cleanroom. The refrigeration skid, vacuum skid, control cabinet and other support equipment are remotely mounted in the unclassified mechanical space either on a mezzanine or behind the wall of the cleanroom.
Can component tubs or liquid-filled vials be transported through the ALUS™ system?
Yes. When the ALUS™ is not used for loading or unloading the lyophilizer, transportation of tubs or liquid-filled vials through the PSI-L isolator is easily accomplished. The conveyor belt and associated tooling can be easily configured to allow tubs of syringes to pass through the isolator. Vials that have been liquid-filled and stoppered may also be transported through the ALUS™ system and into the aluminum seal crimping module. Liquid-filled vials and component tubs can be transported through the ALUS™ isolator while the lyophilizer cycle is in process or during other lyophilizer preparation activities.
What kind of freeze dryers are available from GEA?
The LYOVAC™ state-of-the-art lyophilizers and ALUS™ Automatic Loading and Unloading Systems have been specifically developed to fit into the VarioSys®® concept. GEA can deliver standard sizes for the VarioSys®® with a usable shelf area of 4.5 m² or 11 m². These smaller systems are built to comply with cGMPs and meet the same exacting standards as the larger production equipment offered by GEA. Shelf and chamber capacity is as follows FCM 75-I and FCM 150-I.
I see that GEA is the preferred lyophilizer. Is this sterilized as part of the system or separate? If separate, what is the sterilization cycle?
The ALUS™ system and fascia of the lyophilizer are sealed within a PSI-L isolator and are VHP compatible. The standard VHP decontamination cycle for the PSI-L isolators developed by SKAN can be used for the ALUS™. The lyophilizer chamber/condenser is designed for 1.7 bar @ 130°C and may be sterilized by a traditional steam sterilization cycle of 30 minutes at ≥121°C.
Can the lyophilizer and ALUS™ equipment be added to the VarioSys®® production line at a later time?
Yes. This can be planned during the preliminary design phase of the VarioSys® line and facility. GEA will provide equipment drawings and utility tables for a successful future installation.
Can a quicker time to market be achieved on this kind of equipment versus the large, high-speed industrial lines?
Yes. The standardization of the equipment makes for shorter delivery, documentation and qualification times. The standard design also means this equipment requires less investment than conventional, customized lines.
Are the same features available on these small lyophilizers as the large industrial size lyophilizers? Can scale-up be accomplished in an efficient manner?
Yes. These smaller lyophilizers can be used for clinical scale and small scale commercial manufacturing. If the product requires increased batch sizes in the future, scale-up to the GEA industrial lyophilizers is easily accomplished. For example, the same FALCO (Fully Automated Lyophilizer Control and Operating System) SCADA system is available on any size GEA lyophilizer. The lyophilizer cycle, controls, shelf design, refrigeration performance and other critical performance features are scalable for large industrial size dryers.
Are any PAT options available on these smaller scale lyophilizers?
Yes. Virtually all PAT options available on the industrial size lyophilizers are available on these VarioSys®® models. This includes, for example, the LYOPLUS™ and LYOSPARK™ technologies. LYOPLUS™ is a mass spectrometer based technology that can detect minute amounts of silicone oil within the chamber/condenser. The LYOPLUS™ can also support the basics of lyophilizer cycle optimization and leak detection. LYOSPARK™ is GEA’s controlled nucleation technology which can reduce cycle times by up to 30% and improve the crystalline structure of the product.
Are there any features specific to the GEA lyophilizer to support the freeze drying of potent products?
Yes. Options are available for potent product processing with the lyophilizer. Some of these options include: (1) a filtration system between the condenser and vacuum pumps which prevents accidental release of potent particles through the vacuum system, (2) optional vacuum pump flushing in the event of filter failure, (3) options for CIP recirculation and special draining, (4) spray wands within the PSI-L isolator for wash down of the ALUS™, etc.
How much experience does GEA have with row-by-row loading systems like the one used in the VarioSys® production line?
GEA is one of the most experienced manufactures of systems like this in the world. In 1989 GEA installed its first ALUS™ system. In 1997, the first isolator contained ALUS™ went into operation. Today there are more than 250 GEA ALUS™ installed systems worldwide.
What data are stored from the production run and how is this data transferred?
All individual data and final Portable Document Format (PDF) batch protocols can be transferred to a system at site. The system is 21 CFR Part 11 compliant.
Can the machine be remotely operated through a machine interface, such as a custom application for remote machine operation and monitoring?
The Service Portal is installed as standard for remote access to the machine modules by Bausch+Stroebel and PSI-L from SKAN for troubleshooting purposes. This service is free of charge during the warranty period.
Are all machines controlled by the same SCADA system?
B+S and SKAN have separate processes where machine operation is visualized on a single Human Machine Interface (HMI). GEA has a separate HMI.